Abstract:
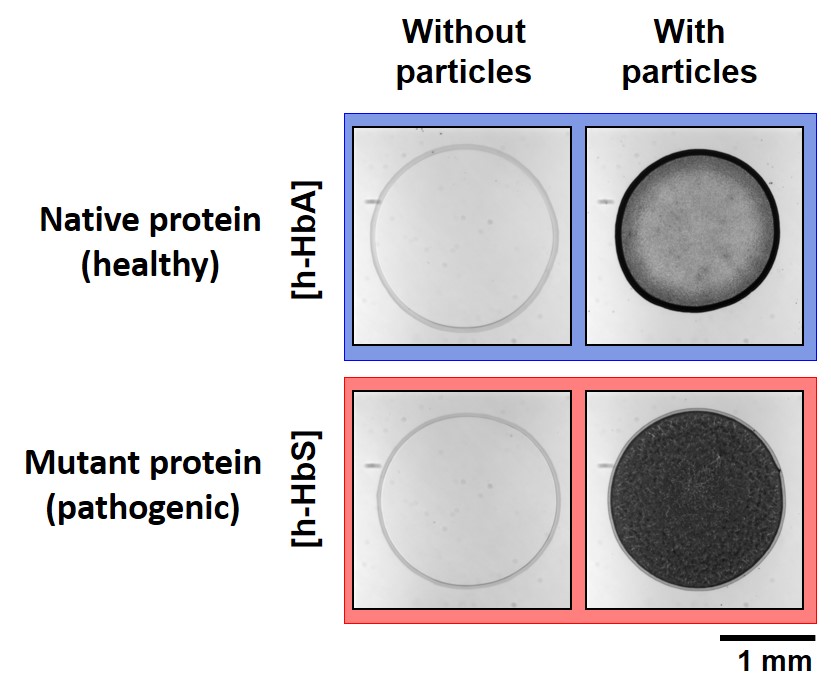
The coffee-ring effect denotes the accumulation of particles at the edge of an evaporating sessile drop pinned on a substrate. Because it can be detected by simple visual inspection, this ubiquitous phenomenon can be envisioned as a robust and cost-effective diagnostic tool. Toward this direction, here we systematically analyze the deposit morphol-ogy of drying drops containing polystyrene particles of different surface properties with various proteins (bovine se-rum albumin (BSA) and different forms of hemoglobin). We show that deposit patterns reveal information on both the adsorption of proteins onto particles and their reorganization following adsorption. By combining pattern analysis with adsorption isotherm and zeta potential measurements, we show that the suppression of the coffee-ring effect and the formation of a disk-shaped pattern is primarily associated to particle neutralization by protein adsorption. How-ever, our findings also suggest that protein reorganization following adsorption can dramatically invert this tendency. Exposure of hydrophobic (resp. charged) residues can lead to disk (resp. ring) deposit morphologies independently of the global particle charge. Surface tension measurements and microscopic observations of the evaporating drops show that the determinant factor of the deposit morphology is the accumulation of particles at the liquid/gas interface dur-ing evaporation. This general behavior opens the possibility to probe protein adsorption and reorganization on parti-cles by the analysis of the deposit patterns, the formation of a disk being the robust signature of particles rendered hydrophobic by protein adsorption. We show that this method is sensitive enough to detect a single point mutation in a protein, as demonstrated here by the distinct patterns formed by human native hemoglobin h-HbA and its mutant form h-HbS, which is responsible for sickle cell anemia.
Reference:
The coffee-ring effect denotes the accumulation of particles at the edge of an evaporating sessile drop pinned on a substrate. Because it can be detected by simple visual inspection, this ubiquitous phenomenon can be envisioned as a robust and cost-effective diagnostic tool. Toward this direction, here we systematically analyze the deposit morphol-ogy of drying drops containing polystyrene particles of different surface properties with various proteins (bovine se-rum albumin (BSA) and different forms of hemoglobin). We show that deposit patterns reveal information on both the adsorption of proteins onto particles and their reorganization following adsorption. By combining pattern analysis with adsorption isotherm and zeta potential measurements, we show that the suppression of the coffee-ring effect and the formation of a disk-shaped pattern is primarily associated to particle neutralization by protein adsorption. How-ever, our findings also suggest that protein reorganization following adsorption can dramatically invert this tendency. Exposure of hydrophobic (resp. charged) residues can lead to disk (resp. ring) deposit morphologies independently of the global particle charge. Surface tension measurements and microscopic observations of the evaporating drops show that the determinant factor of the deposit morphology is the accumulation of particles at the liquid/gas interface dur-ing evaporation. This general behavior opens the possibility to probe protein adsorption and reorganization on parti-cles by the analysis of the deposit patterns, the formation of a disk being the robust signature of particles rendered hydrophobic by protein adsorption. We show that this method is sensitive enough to detect a single point mutation in a protein, as demonstrated here by the distinct patterns formed by human native hemoglobin h-HbA and its mutant form h-HbS, which is responsible for sickle cell anemia.
Reference:
Protein adsorption and reorganization on nanoparticles probed by the coffee-ring effect: application to single point mutation detection
S. Devineau, M. Anyfantakis, L. Marichal, L. Kiger, M. Morel, S. Rudiuk, D. Baigl*
J. Am. Chem. Soc. 2016,138, 11623–11632
![]() -doi : 110.1021/jacs.6b04833
-doi : 110.1021/jacs.6b04833