Abstract:
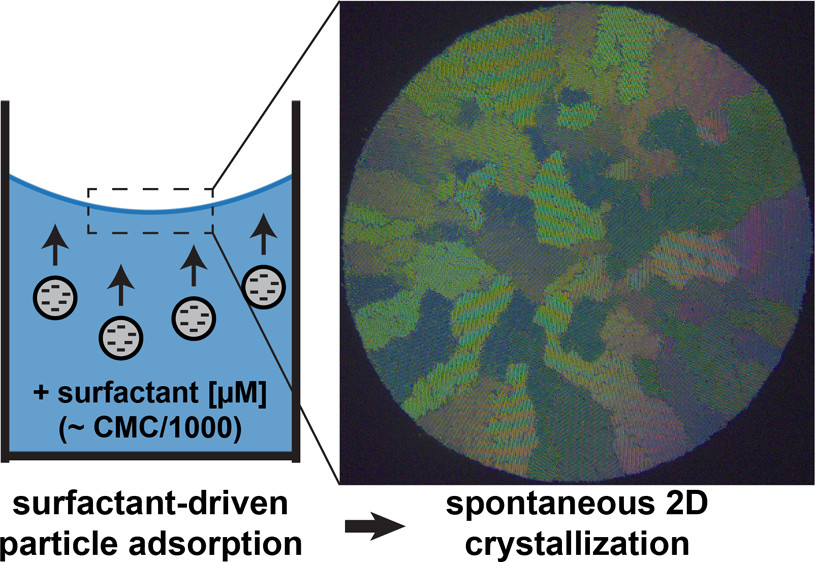
Controlling the organization of particles at liquid–gas interfaces usually relies on multiphasic preparations and external applied forces. Here, we show that micromolar amounts of a conventional cationic surfactant induce, in a single step, both adsorption and crystallization of various types of nanometer- to micrometer-sized anionic particles at the air–water interface, without any additional phase involved or external forces other than gravity. Contrary to conventional surfactant-induced particle adsorption through neutralization and hydrophobization at a surfactant concentration close to the critical micellar concentration (CMC), we show that in our explored concentration regime (CMC/1000-CMC/100), particles adsorb with a low contact angle and maintain most of their charge, leading to the formation of two-dimensional assemblies with different structures, depending on surfactant (Cs) and particle (Cp) concentrations. At low Cs and Cp, particles are repulsive and form disordered assemblies. Increasing Cp in this regime increases the number of adsorbed particles, leading to the formation of mm-sized, highly ordered polycrystalline assemblies because of the long-range attraction mediated by the collective deformation of the interface. Increasing Cs decreases the particle repulsion and therefore the interparticle distance within the monocrystalline domains. A further increase in Cs (≈CMC/10) leads to a progressive neutralization of particles accompanied by the formation of disordered structures, ranging from densely packed amorphous ones to loosely packed gels. These results emphasize a new role of the surfactant to mediate both adsorption and crystallization of particles at liquid–gas interfaces and provide a practical manner to prepare two-dimensional ordered colloidal assemblies in a remarkably robust and convenient manner.
Adsorption and Crystallization of Particles at the Air-Water Interface Induced by Minute Amounts of Surfactants
M. Anyfantakis, J. Vialetto, A. Best, G. K. Auernhammer, H.-S. Butt, B. P. Binks, D. Baigl.
Langmuir 2018, 34, 15526−15536