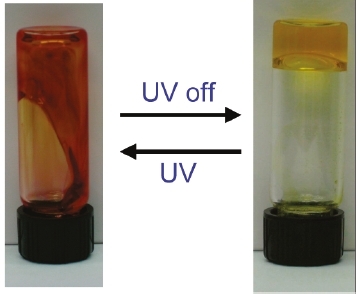
Reference: J. Am. Chem. Soc. 2011, dx.doi.org/10.1021/ja202412z
Title: Reversible Photorheological Fluids Based on Spiropyran Doped Reverse Micelles
Authors: H.-Y. Lee, K. K. Diehn, K. Sun, T. Chen and S. R. Raghavan
Review by: Antoine Diguet
Title: Reversible Photorheological Fluids Based on Spiropyran Doped Reverse Micelles
Authors: H.-Y. Lee, K. K. Diehn, K. Sun, T. Chen and S. R. Raghavan
Review by: Antoine Diguet
This article describes a new photorheological fluid, prepared by certainly the most productive lab in this field.
A photorheological fluid is a fluid whose rheological properties (mainly the viscosity) can be modified by light. This is not the first time that such a medium is presented. It seems that the first demonstration has been proposed in 1989 [1]. The development of photorheological fluid really started in the middle of last decade. In particular, photorheological aqueous solutions were prepared by mixing a photosensitive surfactant (very similar to AzoTAB) and a polyelectrolyte [2] or another surfactant [3]. Even if tens of such fluids now exist (with other photosensitive molecules, for example the trans-ortho-methoxycinnamic acid [4], with possibility of photoinduced gelation [5],…), authors emphasize here the simple preparation of the fluid (made of only three commercially available compounds) and the reversibility of the phenomenon.
A photorheological fluid is a fluid whose rheological properties (mainly the viscosity) can be modified by light. This is not the first time that such a medium is presented. It seems that the first demonstration has been proposed in 1989 [1]. The development of photorheological fluid really started in the middle of last decade. In particular, photorheological aqueous solutions were prepared by mixing a photosensitive surfactant (very similar to AzoTAB) and a polyelectrolyte [2] or another surfactant [3]. Even if tens of such fluids now exist (with other photosensitive molecules, for example the trans-ortho-methoxycinnamic acid [4], with possibility of photoinduced gelation [5],…), authors emphasize here the simple preparation of the fluid (made of only three commercially available compounds) and the reversibility of the phenomenon.
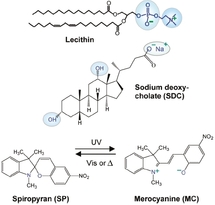
The composition of the fluid is the following : 100mM lecithin, 35mM SDC, and 15 mM SP, in cyclohexane (see Figure). Lecithin is a phospholipid that forms inverse micelles in organic solvent such as cyclohexan. SDC is a small hydrophobic molecule derived from cholesterol. It contains two hydroxyl groups. SP is a spiropyran, a photochromic compound that photoisomerizes under illumination: the cycle opens after irradiation under UV light, and closes under visible light. The spiropyran function is one of the most common photochromic groups (with diazobenzen) and has been used since a long time for the development of variable shade lenses and memories made of organic material <b>[6]</b>.
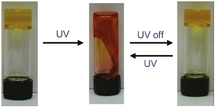
Authors observed that when the liquid is exposed under UV light during 5 minutes, its viscosity decreases (about 10 times smaller). The viscosity goes back to the initial value after 10 minutes under ambient light (see Figure). A more detailed analysis (but very usual for this field) by dynamic frequency spectra of viscous and elastic moduli has been performed. The kinetics of spriropyran photoisomerization has also been characterized by UV/Vis spectroscopy.
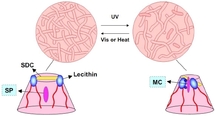
How to explain this photodependent property? It is related to the morphology of micelles present in the fluid (see Figure). In cyclohexan, the surfactant lecithin forms inverse micelles. In the presence of SDC, there are interactions between the headgroup of lecithin and hydroxyl groups of SDC. The resultant micelles have a wormlike shape. These long cylinders entangle and form a transient network which increases the viscosity of the medium. When spiropyran is closed (under visible light), it is a quite apolar molecule that is well solubilized in the organic solvent and stays in the vicinity of micelles.
After UV illumination, spyropyran is in open form which is a zwitterionic molecule and thus had a much less solubility in cyclohexan. Simultaneously, the molecule has a higher affinity for the head group of lecithin (which is also zwitterionic). It can be seen as if spiropyran were solubilized inside lecithin micelles. This modification of the self-assembly favours the growth of shorter structures. The micelle network is thus shorter, the associated viscosity decreases. This modification of the morphology of micelles is usually characterized by small angle neutron scattering (SANS). The transition described above is very common for most of these fluids.
After UV illumination, spyropyran is in open form which is a zwitterionic molecule and thus had a much less solubility in cyclohexan. Simultaneously, the molecule has a higher affinity for the head group of lecithin (which is also zwitterionic). It can be seen as if spiropyran were solubilized inside lecithin micelles. This modification of the self-assembly favours the growth of shorter structures. The micelle network is thus shorter, the associated viscosity decreases. This modification of the morphology of micelles is usually characterized by small angle neutron scattering (SANS). The transition described above is very common for most of these fluids.
This article presents a system that is very easy to prepare, which is quite robust (still reversible after several UV/visible cycles) and whose response time is fast (~5 min). Describing a complex fluid that can be reproduced by anyone, without synthesis step, is interesting to be used by scientists from other fields.
From my point of view, this is not the first time that a fully reversible photorheological fluid is demonstrated (already been done in 2005 [3]). The change of viscosity shown here (factor 10) is actually very low compared to what can be obtained with previous systems (up to 105!). Moreover, this strategy of development of photoresponsive fluid made of simple commercial products has already been (nicely) performed by the same group [7]. A last thing is, for the presentation: on the graphical abstract, I don’t think that the fluid can goes up after visible illumination! The tube has been turned upside down and this has not been mentionned.
From my point of view, this is not the first time that a fully reversible photorheological fluid is demonstrated (already been done in 2005 [3]). The change of viscosity shown here (factor 10) is actually very low compared to what can be obtained with previous systems (up to 105!). Moreover, this strategy of development of photoresponsive fluid made of simple commercial products has already been (nicely) performed by the same group [7]. A last thing is, for the presentation: on the graphical abstract, I don’t think that the fluid can goes up after visible illumination! The tube has been turned upside down and this has not been mentionned.
Another question is: why are these photorheological fluids interesting? Authors (form this team and the others) say that it could be exploited in microfluidics (to be used as valve or flow sensor), in heating and cooling systems (to control flows by light), for controlled release of substances (dyes, perfumes) or for other applications (drag-reducing fluids, ink in inkjet printers, robotics). These medium are well-known since one decade, but for the moment, they have never been used in commercial products or applications. Using them in microfluidics could effectively be interesting: changing fluid viscosity can have dramatic effect on the diffusion of molecules, mixing rates, sorting rates, size of emulsions and multiple emulsions.
References
[1] T. Wolff, C. S. Emming, T. A. Suck, G. Von Bunau. J. Phys. Chem. 1989, 93, 4894-4898
[2] C. T. Jr. Lee, K. A. Smith and T. A. Hatton. Macromolecules 2004, 37, 5397-5405
[3] H. Sakai, Y. Orihara, H. Kodashima, A. Matsumura, T. Ohkubo, K. Tsuchiya and M. Abe. J. Am. Chem. Soc. 2005, 127, 13454-13455
[4] A. M. Ketner, R. Kumar, T. S. Davies, P. W. Elder and S. R. Raghavan. J. Am. Chem. Soc. 2007, 129, 1553-1559
[5] K. S. Sun, R. Kumar, D. E. Falvey and S. R. Raghavan. J. Am. Chem. Soc. 2009, 131, 7135-7141
[6] G. Berkovic, V. Krongauz and V. Weiss. Chem. Rev. 2000, 100, 1741-1753
[7] R. Kumar, A. M. Ketner and S. R. Raghavan. Langmuir 2010, 26, 5405-5411
[1] T. Wolff, C. S. Emming, T. A. Suck, G. Von Bunau. J. Phys. Chem. 1989, 93, 4894-4898
[2] C. T. Jr. Lee, K. A. Smith and T. A. Hatton. Macromolecules 2004, 37, 5397-5405
[3] H. Sakai, Y. Orihara, H. Kodashima, A. Matsumura, T. Ohkubo, K. Tsuchiya and M. Abe. J. Am. Chem. Soc. 2005, 127, 13454-13455
[4] A. M. Ketner, R. Kumar, T. S. Davies, P. W. Elder and S. R. Raghavan. J. Am. Chem. Soc. 2007, 129, 1553-1559
[5] K. S. Sun, R. Kumar, D. E. Falvey and S. R. Raghavan. J. Am. Chem. Soc. 2009, 131, 7135-7141
[6] G. Berkovic, V. Krongauz and V. Weiss. Chem. Rev. 2000, 100, 1741-1753
[7] R. Kumar, A. M. Ketner and S. R. Raghavan. Langmuir 2010, 26, 5405-5411