
Reference: J. Am. Chem. Soc. 2011, dx.doi.org/10.1021/ja2004736
Title: Controlling and Switching the Morphology of Micellar Nanoparticles with Enzymes
Authors: T.-H. Ku, M.-P. Chien, M. P. Thompson, R. S. Sinkovits, N. H. Olson, T. S. Baker and N. C. Gianneshi
Review by: Antoine Diguet
Title: Controlling and Switching the Morphology of Micellar Nanoparticles with Enzymes
Authors: T.-H. Ku, M.-P. Chien, M. P. Thompson, R. S. Sinkovits, N. H. Olson, T. S. Baker and N. C. Gianneshi
Review by: Antoine Diguet
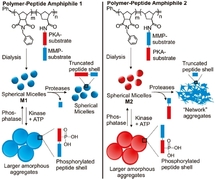
This article describes the possibility of switching the morphology of micellar assembly by enzyme addition. Authors (from University of California) prepared amphiphilic block-copolymer with peptides that represent a substrate for different enzymes. These macromolecules are able to self-assemble in aqueous solution and at the same time, their chemical structure can be modified in the presence of enzymes.
This paper is more than a proof of concept of enzyme-sensitive micelles: authors want to show that the micellar morphology can be modulated by different enzymes, if possible by a reversible manner. To prepare such micelles, the polar head group of copolymers is composed of two regions, sensitive to four different enzymes (protein kinase, protein phosphatase for the first region and matrix-metalloproteinase MMP-2 and MMP-9 for the second one).
This paper is more than a proof of concept of enzyme-sensitive micelles: authors want to show that the micellar morphology can be modulated by different enzymes, if possible by a reversible manner. To prepare such micelles, the polar head group of copolymers is composed of two regions, sensitive to four different enzymes (protein kinase, protein phosphatase for the first region and matrix-metalloproteinase MMP-2 and MMP-9 for the second one).
Due to their typical size (~100 µm), these aggregates were observed by cryo-TEM and SEM techniques. Enzymes have various effects on the structure. A phosphorylation of serine residues is performed by kinase, which increases the polarity of the surface and leads to an increase of aggregate radius (50 times higher!). A very interesting possibility is that this morphology transition is reversible, using dephosphorylation of serine residues by phosphatase. MMP enzymes have an irreversible effect on copolymers: they cleave Gly-Leu bond of peptide. Depending on the position of the Gly-Leu bond in the hydrophilic head, the resulting micellar structure is different. If it is localized at the end of the chain, no sensible modification is observed. If it is localized near the hydrophobic part, the micelle is dramatically converted into an amorphous network, since hydrophilic residues are dispersed in the solution. Despite clear results, these modifications are not immediate and necessitate around 24 h of reaction time.
This is not the first time that such enzyme-sensitive assemblies are realized, but it is a very recent subject. This has been done with micelles that can be destroyed in the presence of enzymes, after cleavage of amphiphilic subunits [1]. The opposite has been done, with block-copolymers that are modified from a water soluble state to a self-assembling state (micelle) in the presence of enzyme [2]. Systems from this new article represent a new step toward the full modulation of micelles/aggregates structures with enzymes. This paper is also a nice illustration of the use of peptides as attractive synthons for the development of responsive supramolecular biomaterials.
Why are this kind of objects interesting? On the one hand, for new detection strategy of diseases. Lots of pathologies are characterized by expression of particular enzymes. Enzymes used in this article are associated with certain cancers. Having small objects that interact with sick regions and give an observable answer, is a way to localize and identify diseases. On the other hand, these objects represent new containers for drug delivery. While transporting biologically active molecules, they could be selectively destroyed and release their content in a specific place.
For instance, enzyme-sensitive assemblies are restricted to micelles. The ideal case would be to create vesicles that can be destroyed while interacting with an enzyme. On the contrary to micelles, vesicles can contain a large amount of water-soluble molecules. Having such objects is very exciting in the frame of the research work in our lab concerning light-induced gene expression. The vesicle could be destroyed, not only by an added external enzyme, but also by an internal expressed enzyme. This would be a nice strategy for the self-release of the content of the vesicle after an appropriate expression time. Ideas for the building of such vesicles can be found in this exhaustive review concerning the controlled self-assembly of supramolecular materials [3].
References
[1] M. A. Azagarsamy, P. Sokkalingam and S. Thayumanavan, J. Am. Chem. Soc. 2009, 131, 14184-14185
[2] R. J. Amir, S. Zhong, D. J. Pochan and C. J. Hawker, J. Am. Chem. Soc. 2009, 131, 13949-13951
[3] Y. Wang, H. Xu and X. Zhang, Adv. Mater. 2009, 21, 2849-2864
[1] M. A. Azagarsamy, P. Sokkalingam and S. Thayumanavan, J. Am. Chem. Soc. 2009, 131, 14184-14185
[2] R. J. Amir, S. Zhong, D. J. Pochan and C. J. Hawker, J. Am. Chem. Soc. 2009, 131, 13949-13951
[3] Y. Wang, H. Xu and X. Zhang, Adv. Mater. 2009, 21, 2849-2864