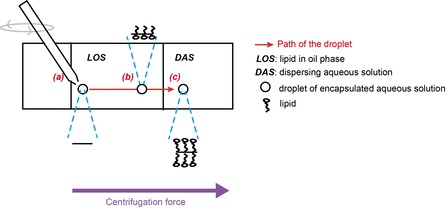
Steps in the vesicle formation: (a) Droplet production (b) Saturation of the droplet surface by lipids in the lipid-in-oil solution (c) Vesicle formation after interface crossing from LOS to DAS
Reference: Soft Matter. 2011, doi: 10.1039/C1SM05239J
Title: Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design
Authors: Manouk Abkarian, Etienne Loiseau and Gladys Massiera
Review by: Anna Venancio
Title: Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design
Authors: Manouk Abkarian, Etienne Loiseau and Gladys Massiera
Review by: Anna Venancio
A new method for vesicles formation is described, by closely monitoring droplets crossing an interface. Vesicles are obtained at a high frequency with good yields. They have a narrow size distribution, tunable on a range of 10 to 40 μm. The technique has a good reproducibility and a great encapsulation efficiency (low amounts of solution needed, and a wide scope of encapsulation candidates).
1. The experimental set-up
A dispersing aqueous solution (DAS), a lipid-in-oil solution (LOS) and an encapsulated aqueous solution (EAS) injected through a capillary, are all submitted to a centrifugation force.
The DAS and LOS are not miscible and form a vertical interface (perpendicular to the centrifugation force). This interface is crossed by the droplets of the EAS, whose movement is also driven by the centrifugation force, to give vesicles. Since centrifugation is the main driving force, the EAS must have a higher density than the DAS, which itself need to have a higher density than the LOS.
A dispersing aqueous solution (DAS), a lipid-in-oil solution (LOS) and an encapsulated aqueous solution (EAS) injected through a capillary, are all submitted to a centrifugation force.
The DAS and LOS are not miscible and form a vertical interface (perpendicular to the centrifugation force). This interface is crossed by the droplets of the EAS, whose movement is also driven by the centrifugation force, to give vesicles. Since centrifugation is the main driving force, the EAS must have a higher density than the DAS, which itself need to have a higher density than the LOS.
2. The steps and the way to monitor their individual parameters
The cDICE method is a fast way of obtaining vesicles because the three main steps leading to vesicle formation happen directly one after the other in the same experimental set-up. The method owes its efficiency to the fact that each step remains tunable individually.
a) The production of droplets is obtained when the EAS is detached from the capillary by the effect of viscosity from the non-aqueous phase (LOS or decane). The droplets formed are monodisperse and their size can be controlled by the capillary diameter and the dapillary number at the tip. Clogging of the capillary sets the minimum size limit that can be reached.
b) The droplet enters the LOS and is coated by the free lipid in solution. The time the droplet spends in the LOS (known as time of flight tf) must be much higher than the time need for lipids to cover the surface of the droplet (ts). Adapting the thickness of the LOS (increase tf) or changing the lipid concentration in LOS (lowering ts) can help make sure this condition (tf >> ts) is respected. This sets an upper boundary for size of vesicles, as larger vesicles have lower tf. Modifying the set-up is necessary to push the limit upwards.
c) The interface crossing, made possible by the application of a centrifugal force, must be controlled in order to obtain of a monolayer membrane without any oil. A phenomena called zipping must happen, where the lipids at the interface form a monolayer with the lipids covering the droplet. The lipid-covered droplet surface should not fuse with the SOL/DAS interface to form a hole, resulting in the contents of the droplet being diluted in the DAS. This second phenomenon explains why the yield of this method is sometimes as low as 40%. Using centrifugation and a slightly higher density of the EAS compared to the DAS also allows a detachment step to occur.
The cDICE method is a fast way of obtaining vesicles because the three main steps leading to vesicle formation happen directly one after the other in the same experimental set-up. The method owes its efficiency to the fact that each step remains tunable individually.
a) The production of droplets is obtained when the EAS is detached from the capillary by the effect of viscosity from the non-aqueous phase (LOS or decane). The droplets formed are monodisperse and their size can be controlled by the capillary diameter and the dapillary number at the tip. Clogging of the capillary sets the minimum size limit that can be reached.
b) The droplet enters the LOS and is coated by the free lipid in solution. The time the droplet spends in the LOS (known as time of flight tf) must be much higher than the time need for lipids to cover the surface of the droplet (ts). Adapting the thickness of the LOS (increase tf) or changing the lipid concentration in LOS (lowering ts) can help make sure this condition (tf >> ts) is respected. This sets an upper boundary for size of vesicles, as larger vesicles have lower tf. Modifying the set-up is necessary to push the limit upwards.
c) The interface crossing, made possible by the application of a centrifugal force, must be controlled in order to obtain of a monolayer membrane without any oil. A phenomena called zipping must happen, where the lipids at the interface form a monolayer with the lipids covering the droplet. The lipid-covered droplet surface should not fuse with the SOL/DAS interface to form a hole, resulting in the contents of the droplet being diluted in the DAS. This second phenomenon explains why the yield of this method is sometimes as low as 40%. Using centrifugation and a slightly higher density of the EAS compared to the DAS also allows a detachment step to occur.
3. Advantages
Among the main advantages of the cDICE method are:
- the very high number of candidates for encapsulation (colloids, blood cells, proteins, high ionic strength or viscous solutions…). Very little amounts of EAS can be used. The main limitations being that the contents of the droplet must not hinder the saturation step by lipids, and that the interaction between the EAS and DAS have to be compatible with the formation of a monolayer
- the very narrow size distribution, for various vesicle sizes over a certain range (10 to 40 um). Increasing that range leads to a drop of monodispersity.
- the low amounts of oil incorporated in the membrane (not precisely measured), which make cDICE vesicles great candidates as biomimetic compartments and encapsulation vectors.
- the wider range of lipids that can be used, and that can most likely be extended to include polymers
Among the main advantages of the cDICE method are:
- the very high number of candidates for encapsulation (colloids, blood cells, proteins, high ionic strength or viscous solutions…). Very little amounts of EAS can be used. The main limitations being that the contents of the droplet must not hinder the saturation step by lipids, and that the interaction between the EAS and DAS have to be compatible with the formation of a monolayer
- the very narrow size distribution, for various vesicle sizes over a certain range (10 to 40 um). Increasing that range leads to a drop of monodispersity.
- the low amounts of oil incorporated in the membrane (not precisely measured), which make cDICE vesicles great candidates as biomimetic compartments and encapsulation vectors.
- the wider range of lipids that can be used, and that can most likely be extended to include polymers
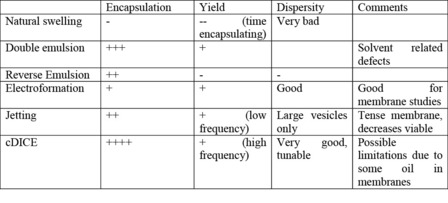
4. How does this method compare to others?